With the continued development of renewable energies in 2025, battery storage for domestic use is expanding at a rapid rate. Two battery technologies continue to vie for dominance in this arena: lead-acid vs. lithium-ion.
These battery chemistries are commonly used for different applications. Lead-acid batteries have been around for over a century and are widely used in automobiles, motorcycles, and backup power systems. Conversely, lithium-ion batteries are relatively new and are commonly used in consumer electronics, electric vehicles, and renewable energy systems.
Both types of batteries have advantages and disadvantages, making them suitable for different applications based on specific requirements such as power output, life cycle, cost, and environmental impact.
In this article, we take a closer at lead-acid and lithium-ion batteries by discussing 10 key differences between the two technologies.
Moreover, we look at the future of both technologies.
Table of Contents
Lead-acid vs. lithium-ion: How do they work?
Lead-acid and lithium-ion batteries share the same working principle based on electrochemistry. They store (charge) and release (discharge) electrons (electricity) through electrochemical reactions.
Both of them feature the following parts:
- Two electrodes: Anode (-), and Cathode (+).
- Electrolyte.
- Membrane separator.
They differ in the material used for each component:
| Lead-Acid | Lithium-Ion | |
|---|---|---|
| Anode | Pb | Carbon |
| Cathode | PbO2 | Lithium Oxide (LiFePO4, LiCoO2, LiMn2O4, etc…) |
| Electrolyte | H2SO4 (liquid, gel) | Lithium salt (liquid, solid, gel) |
The following diagram illustrates the working principle of a lithium battery during discharging and charging. (This principle also applies to lead-acid batteries):
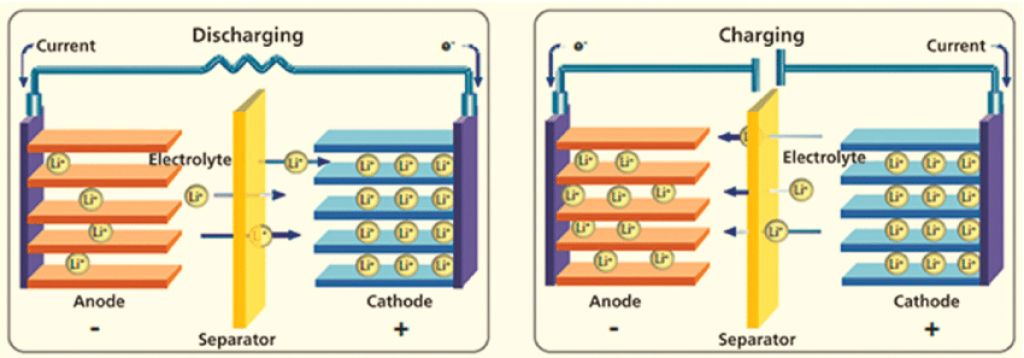
While discharging, Lithium ions (Li+) move between the negative electrode (anode) and the positive electrode (cathode).
Electrons are released from the negative to the positive electrode to balance the reaction.
While charging, the opposite reaction occurs, and electrons flow from the positive electrode to the negative.
A similar reaction occurs in lead-acid batteries, but in this instance, the acid electrolyte (H2SO4) participates in the reaction with 2H+(aqueous protons).
Lead-acid vs. lithium-ion: Which one has better capacity?
From a microscopic point of view, a battery’s capacity relates to the global charge of the transferred ions (Li+ or H+) multiplied by the working voltage of the electrochemical reaction.
Herein lies the primary difference between lead-acid and lithium-ion technologies — weight.
Lithium is the lightest metal on earth. One kg of lithium contains 29 times more atoms than lead. In addition, the working voltage of Lithium-Ion is 3.2V vs. 2V for lead-acid. Consequently, you can store much more energy in 1kg of lithium battery than in lead-acid.
The chart below summarizes the energy storage capacity of both technologies. The theoretical density does not consider the mass of the electrolytes and other components (battery casing, safety equipment…).
| Lead-Acid | Lithium-Ion | |
|---|---|---|
| Storage capacity theory | 167 Wh/kg | 11,600 Wh/kg |
| Storage capacity practice | 30–40 Wh/kg | 110–250 Wh/kg |
Two other interesting figures are the battery capacity, also called energy density in Wh/l, and the specific power of the battery in W/kg.
| Lead-Acid | Lithium-Ion | |
|---|---|---|
| Energy density | 80-90 Wh/l | 250 – 670 Wh/l |
| Specific power | 180 W/kg | 250 – 340 W/kg |
Ultimately, lithium batteries have the following advantages over their lead-acid counterparts:
- Lighter: A lithium-ion battery with the same capacity as a lead-acid one can be six times lighter.
- More compact.
- Twice as powerful.
Lead-acid vs. lithium-ion: Can you fully discharge them?
Maximum depth of discharge (DOD)
Lead-acid
Those of you with lead-acid batteries may have noticed that the manufacturer advises not to discharge the battery below 50% of its full capacity to improve its life duration.
In lead-acid batteries, over-discharging creates parasite reactions (sulfation) at the electrodes, slowly damaging the system.
Researchers and engineers worked hard to remedy this matter by introducing Gel and AGM batteries. These variants offer a better depth of discharge (DOD) than traditional flooded lead-acid batteries. That said, fully discharging these batteries still affects them negatively.
Lithium-ion
Conversely, lithium battery degradation only occurs when the DOD reaches 60%. As such, manufacturers recommend 80% DOD to improve their total life duration.
That said, recent improvements in lithium technology enable 100% DOD without damaging the battery.
Self-discharge rate
When stored, both technologies will slowly lose their initial capacity. Why? Because a battery’s initial state of charge affects the discharge rate. As such, it’s essential that you charge your battery fully before storing it.
The self-discharge rate for lead-acid batteries is 3-20% a month and 0.35-2.5% per month for lithium-ion batteries.
Charge/discharge efficiency (round-trip efficiency)
The charge efficiency reflects the actual quantity of energy effectively stored in the battery. For example, when charging a 1 kWh battery, you might use more than 1 kWh due to internal loss.
Generally speaking, your average lithium-ion battery will lose approximately 5-10% of energy during a round trip, compared to 20-25% for lead-acid batteries.
Lead-acid vs. lithium-ion: Which is more durable?
A product’s durability heavily influences its worth. The same applies to batteries. Unfortunately, this is not a strength of lead-acid batteries.
That said, some improvements have been made to lead-acid batteries over the years. Many have become maintenance-free, and AGM and GEL technologies were introduced that slightly improved their performance.
With only two years of good service — when discharged at 50% of its maximum capacity — their life duration is extremely low, especially for a century-old technology. For instance, if you deep discharge your lead-acid battery each cycle — at 80% or more — it’ll only work well for 350 cycles or one year.
Conversely, the lifespan of lithium batteries has risen over the last five years. Manufacturers now offer warranties as high as 10,000 cycles or 10 years (70% of initial capacity).
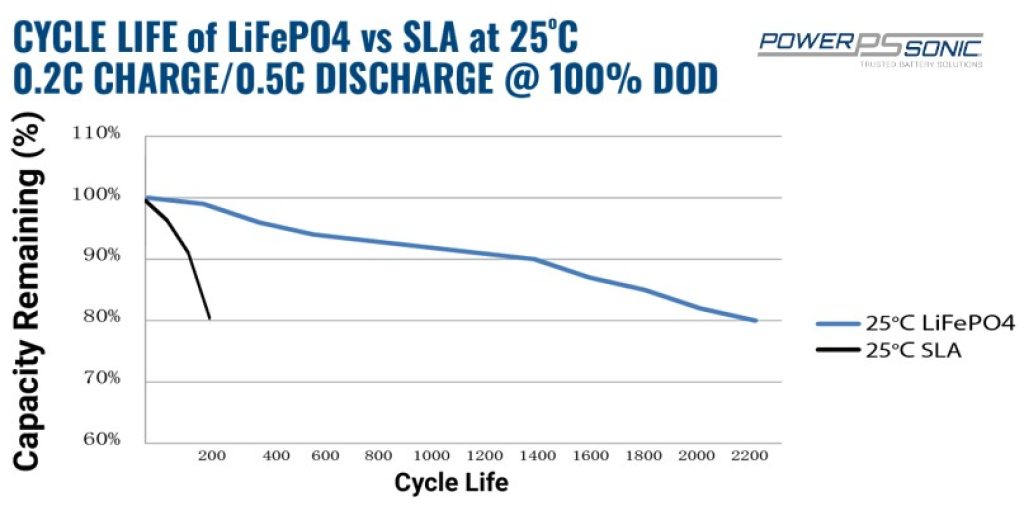
Source: Power Sonic
Related reading: Lithium Battery Cycle Life
Lead-acid vs. lithium-ion: Do they support quick charge/discharge?
Due to technical limitations, lead-acid batteries don’t support quick charging. Their charging time range from 6-15 hours and follow a three-step process:
- Bulk charge: The charging voltage increases steadily to its maximum value, and the charging current is kept at its maximum value. The battery will reach up to 80% of its full capacity in approximately 6-8 hours.
- Absorption charge: The charging voltage is kept at its maximum value while the current slowly decreases until the battery is charged at 90-95%.
- Float charge: Charging voltage and current decrease to zero while the battery reaches full charge.
Conversely, lithium batteries are fit for a quick charge. You can charge them to 80% of their full capacity in 1-2 hours (depending on the power output of your charger).
The remaining 20% will take another 2-3 hours. Therefore, you can fully charge a lithium-ion battery in 3-5 hours.
Both types of batteries support quick discharge and can provide intense pulses of current (hundreds of amps) if required.
Lead acid vs. lithium-ion: Which is the best for solar energy?
Solar energy, like all renewables, is intermittent. Therefore, its power output varies depending on the time of the day and the weather.
Passing clouds generate drastic power output modifications that affect a battery’s charging current.
As previously mentioned, lead-acid batteries can be damaged by these occurrences before they follow the three-step process for proper charging.
In addition, their charging time can take up to 15 hours, and in most countries, days are shorter than 15 hours, meaning these batteries will never reach full charge.
Conversely, intermittent charging does not affect lithium-ion batteries as they can reach full charge within a few hours. Consequently, lithium batteries are far better suited to solar energy storage than lead-acid batteries.
Related reading: What are the 7 best storage batteries for solar panels in 2025?
Lead-acid vs. lithium-ion: Are they safe?
Lead-acid and lithium-ion contain hazardous materials that can harm their owners and the environment.
To make their products safe, manufacturers have developed different strategies summarized in the chart below:
| Risk | Safety Equipment | |
|---|---|---|
| Lead-acid | Spilling of acidic electrolyte | The electrolyte as a Gel or absorbed on a glass mat (AGM) |
| Release of highly toxic H2S gas | Valve Regulation | |
| Lithium-Ion | Fire/explosion | Airtight packaging |
| Battery Management Systems (BMS) | ||
| Solid electrolyte |
Most of the lead-acid batteries available on the market are now Valve Regulated (VRLA) and maintenance-free, therefore safe to use.
Pure lithium is highly flammable when in contact with air. As such, battery management systems (BMS) were developed for safety purposes to monitor each battery cell and ensure no overcharge/discharge.
Ultimately, both batteries are safe to use, but they still need to be handled with caution.
Related reading: A Safety Guide To Swollen Lithium Batteries
Lead-acid vs. lithium-ion: Which is cheaper?
The Levelized Cost of Storage (LCOS) is the best way to compare the cost of different battery technologies. LCOS is expressed in USD/kWh and considers all the expenses related to energy storage over the lifespan of a battery.
As batteries are maintenance-free, the only cost would be the price of the battery itself.
Take a look at the following example:
| Expert Power | Expert Power | Renogy | |
|---|---|---|---|
| Battery type | LiFePO4 | VLRA | Hybrid Gel |
| Capacity | 100Ah (1.2 kWh) | 100Ah (1.2 kWh) | 100Ah (1.2 kWh) |
| Number of cycles at DOD | 3600 cycles @ 80% DOD | 500 cycles @ 50% DOD | 750 cycles @ 50% DOD |
| Total kWh over lifetime | 3,456 kWh | 300 kWh | 450 kWh |
| Total cost | $399.99 | $139.99 | $206.99 |
| LCOS | 0.115 $/kWh | 0.466 $/kWh | 0.459 $/kWh |
From the table above, it’s obvious that the initial cost of the lithium-ion battery (LiFePO4) is higher. However, as time goes on, its superior cycle life results in it being four times cheaper than its lead-acid rivals.
Lead-acid vs. lithium-ion: Can you recycle them?
As previously mentioned, both batteries contain hazardous, highly toxic material and should be disposed of properly.
Lead-acid batteries are the most recycled product in the world. The recycling rate reaches almost 100% in the U.S. as nearly all parts (sulfuric acid electrolyte, ABS casing, lead plates) can be recycled. Additionally, new lead-acid batteries usually contain more than 80% recycled material.
Conversely, lithium-ion is a new technology; recycling this material still proves to be challenging. However, as our article discussing recycling lithium batteries shows, it is possible.
Lead-acid vs. lithium-ion: Can you DIY?
Lead-acid batteries are sealed. As such, you cannot upgrade and/or modify them.
Thankfully, this is not the case with lithium-ion batteries. These batteries are modular, meaning you can build your very own battery pack at home.
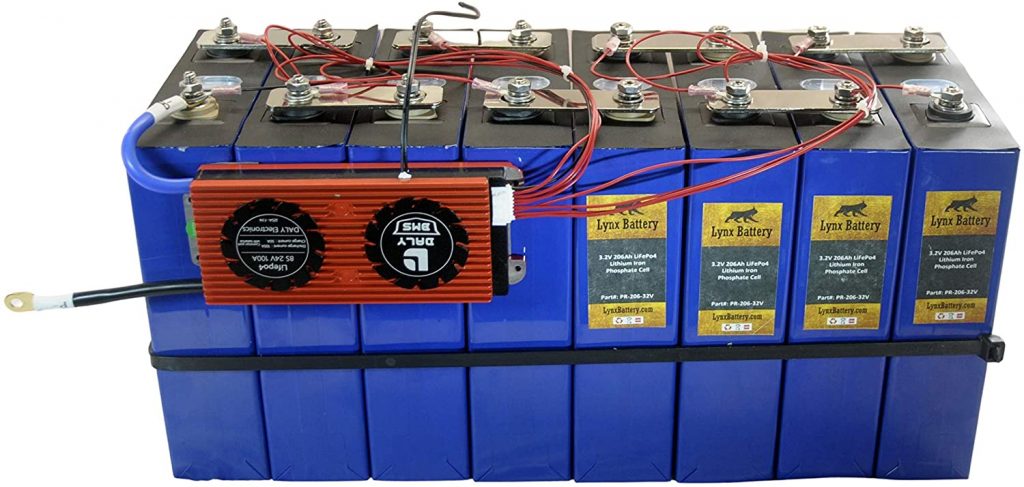
When purchasing a lithium battery, you’re buying multiple battery cells assembled together. All of which are connected to the battery management system (BMS).
All elements constituting the lithium battery pack can be bought individually online.
Manufacturers are selling prismatic lithium battery cells of various capacities (from 10Ah to 300Ah), all rated at 3.2V.
For example, you can purchase four prismatic cells of 200Ah capacity each. Mount them in series, connect all the prismatic cells to a BMS, and you’ll get a 12V, 200Ah lithium battery.
This illustrates the great modularity of lithium batteries.
Lead-acid vs. lithium-ion: What does the future hold?
The future of lead-acid batteries
It’s unlikely that lead-acid batteries will become extinct in the near future.
Despite facing significant competition from newer battery technologies, lead-acid batteries still have several advantages that make them an attractive option for specific applications. They are:
- Relatively cheap.
- Have a well-established production and recycling infrastructure.
- Have a proven track record of reliability and performance.
Moreover, the development of advanced lead-acid battery technologies, such as AGM and Gel batteries, has helped to address some of the limitations of traditional flooded lead-acid batteries, such as improved performance and longer cycle life.
Ultimately, lead-acid batteries will probably continue to play a significant role in certain applications where their advantages, such as cost and reliability, make them an attractive option.
The future of lithium-ion batteries
The lithium-ion battery industry is expected to continue its rapid growth, driven by the increasing demand for high-performance and sustainable energy storage solutions.
The demand for EVs is one of the main catalysts for growth in the lithium battery market, as the need for longer-range, faster-charging, and more affordable EVs continues to increase.
Additionally, the continuous development of new and improved battery materials, such as solid-state batteries and silicon anodes, is expected to further increase the performance and safety of these batteries in the future.
These new materials have the potential to significantly increase energy density and reduce the risk of thermal runaway. This condition can cause a lithium-ion battery to catch fire or explode.
In the end, the future of the lithium-ion battery industry looks very promising, with continued growth driven by increasing demand for high-performance and sustainable energy storage solutions.
The development of new and improved battery materials will likely further increase the performance and safety of lithium-ion batteries, making them an even more attractive option for a wide range of applications.
Final thoughts
This is an overwhelming victory for lithium-ion batteries over lead-acid batteries.
Lithium-ion batteries offer larger capacities and are more durable, lightweight, efficient, and cheaper than lead-acid batteries. Furthermore, they are modular, and you can build your battery pack at home.
Thanks to the rapid development of electric vehicles (EVs), lithium battery prices will continue to drop, making this technology even more attractive in the future.