Lithium battery cycle life refers to the number of charge and discharge cycles that a lithium battery can perform before it starts losing performance and its capacity drops to about 80% of its initial capacity.
There are many types of lithium-ion batteries, each with its advantages and disadvantages.
Some can last much longer than others, depending on their chemistry and how well you care for them.
For example, the depth of discharge significantly impacts the cycle life of Li-ion batteries. So if you fully discharge your lithium battery frequently, it will most likely perform fewer cycles than otherwise.
Now, do you know which type of lithium battery has the longest cycle life?
In this article, we’ll answer this question as we look at the cycle life of different types of lithium battery chemistries in detail.
Affiliate Disclaimer
Table of Contents
Lithium Battery Cycle Life Chart
| Type of Lithium Battery | Cycle life |
|---|---|
| Lithium Cobalt Oxide – LCO | Between 500 and 1.000 cycles |
| Lithium Manganese Oxide – LMO | Between 300 and 700 cycles |
| Lithium Nickel Manganese Cobalt Oxide – NMC | Between 1.000 and 2.000 cycles |
| Lithium Iron Phosphate – LFP | Between 2.000 and 4.000 cycles |
| Lithium Nickel Cobalt Aluminum Oxide – NCA | Around 500 cycles |
| Lithium Titanate – LTO | Between 3.000 and 7.000 cycles |
Lithium-Ion Batteries
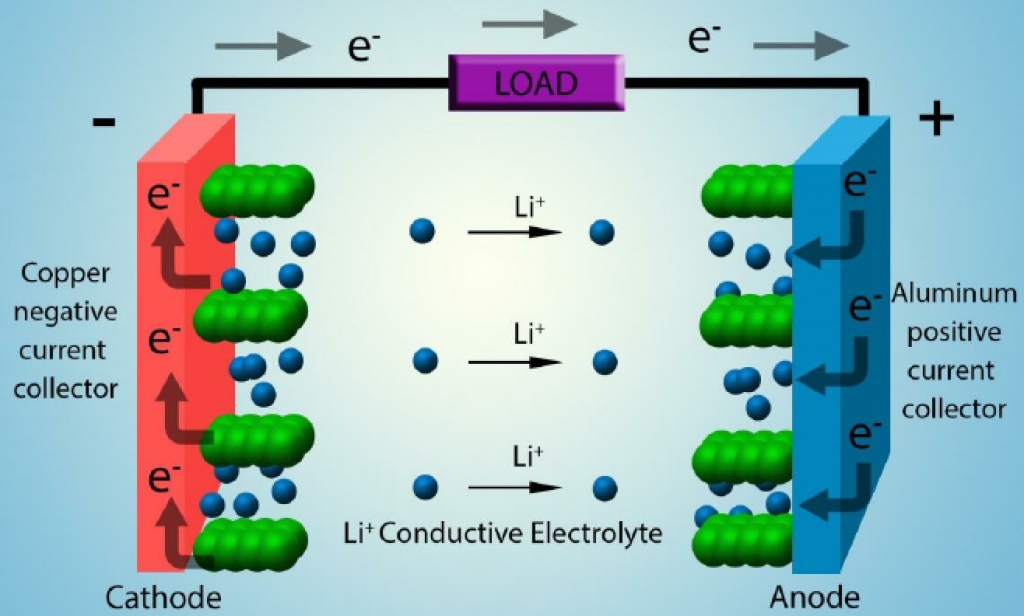
Source: Adapted from “3-D Micro and Nano Technologies for Improvements in Electrochemical Power Devices.”
A typical lithium-ion battery has two electrodes. Each electrode is referred to as either anode or cathode, depending on its role during discharge.
In reality, both electrodes act as anode and cathode. Their roles switch depending on whether the cell is charging or discharging.
Typically, the electrode with higher potential is called the cathode.
The cathode is usually a lithium transition metal (Mn, Ni, Co, Fe, Cr, etc.) oxide, capable of undergoing reversible removal/loss of Li+. It is the cathode that determines the energy density of the battery.
The other electrode — referred to as the anode — is an intercalation material, usually graphite or graphite hybrid material (or, in some cases, lithium titanate (LTO)).
Lithium ions move back and forth between the electrodes in a lithium battery. During discharge, Li+ moves through the electrolyte to the cathode. When charging, the lithium ions move back from the cathode to the anode, again through the electrolyte.
The main difference between the many types of lithium batteries is the materials that make up the cathode and anode.
Consequently, batteries with different electrode materials differ in performance, safety, and durability (cycle life).
The aging mechanism and cycle life of different lithium batteries vary considerably and mainly depend on the cathode and anode materials.
Compared to other types of batteries, lithium-ion batteries are superior. They are reliable, safe, have a high energy density, and last for a very long time. However, lithium batteries can degrade much more quickly if not cared for properly.
How Many Life Cycles Does A Lithium Cobalt Oxide (LCO) Battery Have?
Lithium cobalt oxide batteries usually last between 500 to 1.000 cycles.
The number of cycles varies depending on how you use it/care for it. Why? Because a battery’s cycle life is affected by many different stress factors like temperature, state of charge (therefore, depth of discharge), and charge and discharge current.
LCO batteries are widely used in smartphones, laptops, and digital cameras, given their high energy density. However, they are susceptible to thermal runaway in cases of abuse and can be pretty dangerous if not handled properly. They also have a relatively short cycle life.
LCO Specs
| Cathode | Lithium Cobalt Oxide (LiCoO2) |
|---|---|
| Anode | Graphite |
| Cell Voltage | 3.6V |
| Applications | Smartphones, laptops, cameras, tablets, small gadgets |
| Cycle Life | Between 500 and 1.000 cycles |
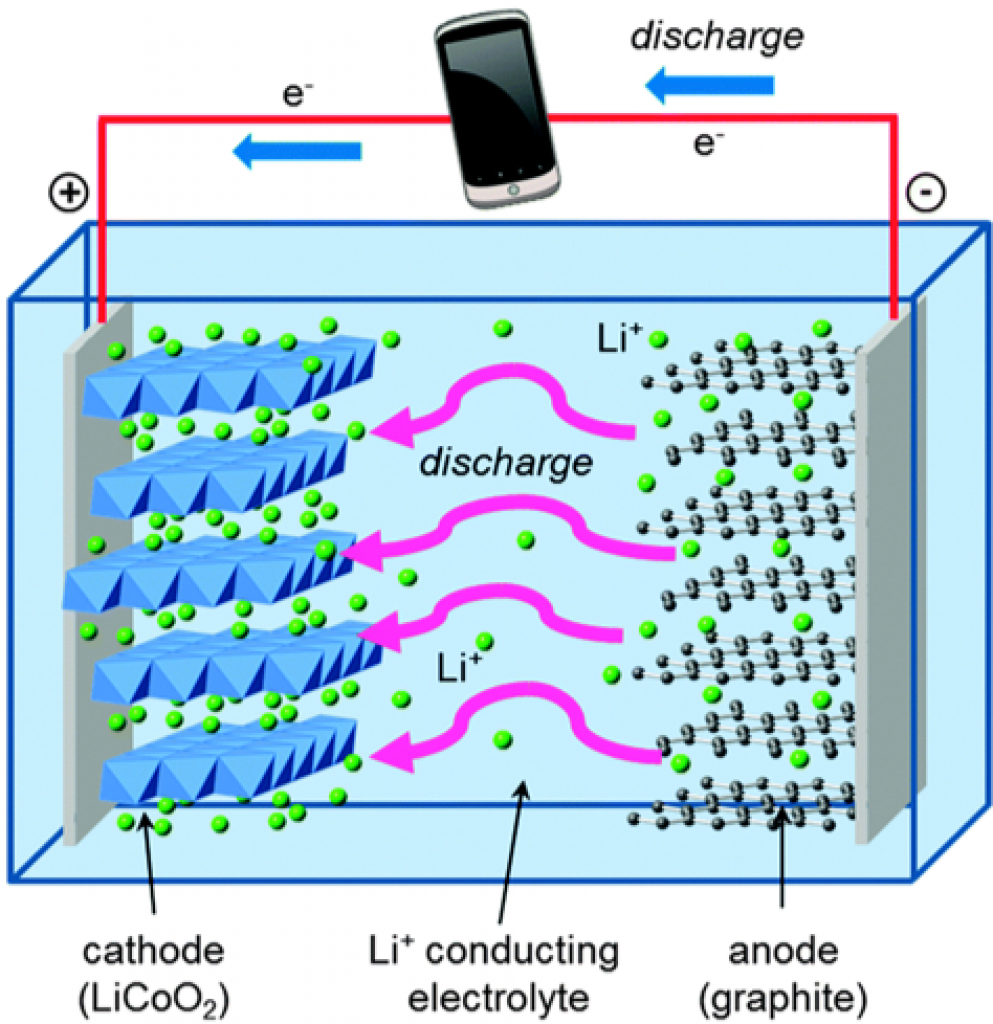
Source: erb.umich.edu
The lithium cobalt oxide’s structure allows for a high energy density. However, LCO batteries don’t last much, and cobalt is relatively expensive. These two facts combined make the LCO battery not very cost-effective.
In addition, stress factors can lead to lithium platting or the growth of resistive layers that can potentially puncture the separator membrane, leading to a short circuit.
This short circuit (or the low thermal stability) can produce a fire or an explosion, so the use of LCO batteries is limited to small devices.
How Many Life Cycles Does A Lithium Manganese Oxide (LMO) Battery Have?
Lithium manganese oxide batteries (LMO) can last from 300 to 700 cycles.
This type of lithium battery is very powerful. It can deliver (and receive) a high current, making it a good battery for power tools, electric powertrains, and medical devices.
The cathode has a three-dimensional spinel structure that facilitates ion flow, resulting in lower internal resistance and improved current handling.
The main drawback is its relatively low cell capacity (roughly one-third of the lithium cobalt oxide battery capacity) and short cycle life.
LMO Specs
| Cathode | Lithium Manganese Oxide (LiMn2O4) |
|---|---|
| Anode | Graphite |
| Cell Voltage | 3.7V |
| Applications | Power tools, medical devices, electric powertrains |
| Cycle Life | Between 300 and 700 cycles |
Its short cycle life is explained by the fact that Manganese can assume several oxidation states (+2, +3, +4, +5, +6, +7). When the average oxidation state of the manganese drops below +3.5, the cathode’s surface suffers degradation because, from the Mn+3.5, Mn+2 and Mn+4 can be formed.
Mn+2 is soluble in most electrolytes, so active material is lost, degrading the cathode.
This and other degradation mechanisms result in a short cycle life for lithium manganese oxide batteries (despite their high current tolerance).
How Many Life Cycles Does A Lithium Nickel Manganese Cobalt Oxide (NMC) Battery Have?
Another type of lithium battery is the lithium nickel manganese cobalt oxide, or simply “NMC.” This type of battery normally performs between 1.000 to 2.000 cycles.
NMC batteries are mainly used mainly in electric cars, including hybrid vehicles.
The NMC is one of the most successful lithium-ion batteries. Why? Because it combines the advantages of each of its components: manganese, nickel, and cobalt.
Nickel is known for its high energy density, but it’s not so stable. On the other hand, Manganese offers a low specific energy, but due to its structure, it facilitates ion flow (given its low internal resistance).
NMC Specs
| Cathode | Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2) |
|---|---|
| Anode | Graphite |
| Cell Voltage | 3.6V |
| Applications | Industrial machinery, e-bikes, medical devices, and EVs |
| Cycle Life | Between 1.000 and 2.000 cycles |
Battery systems that contain nickel tend to have a high energy density, lower cost, and longer cycle life than cobalt-based cells. However, their specific power is lower (they deliver a lower voltage).
The relatively short cycle life results from the changes in the cathode’s structure during cycling. Manganese doesn’t form stable structures as it can assume many different oxidation states.
How Many Life Cycles Does A Lithium Iron Phosphate (LFO) Battery Have?
Lithium iron phosphate battery is a very popular type of lithium battery. This type of battery is widely used as a solar battery, partially due to its long cycle life, ranging from 2000 to 4000 cycles.
LiFePO4 batteries are made of inherently stable materials that don’t deteriorate quickly with use. For this reason, they present a relatively long cycle life.
The drawback is a low energy density (compared to other types of lithium batteries).
LFO Specs
| Cathode | Lithium Iron Phosphate (LiFePO4) |
|---|---|
| Anode | Graphite |
| Cell Voltage | 3.2V |
| Applications | Stationary applications like RV, campervan, boat, off-grid cabin battery bank |
| Cycle Life | 2.000+ cycles |
The LFO cathode structure offers low internal resistance. The benefits from this are a long cycle life and a high current rating. In addition, the stability of this cathode provides a high tolerance for abuse, making it one of the safest lithium-ion batteries.
Nevertheless, LFO batteries can suffer capacity loss caused by structural disorders that damage the electrode. These structural changes are accelerated mainly by increasing discharge rates.
Conversely, at low discharge rates, the main aging factor of LFO batteries is the irreversible loss of active lithium due to the generation of an SEI film.
How Many Life Cycles Does A Lithium Titanate (LTO) Battery Have?
Lithium Titanate batteries offer the longest cycle life of all lithium batteries, ranging from 3000 to 7000 cycles.
This type of lithium battery is different from the others because the anode is not graphite (or silicon); the anode is lithium titanate. As for the cathode, it can be lithium manganese oxide or NMC.
LTO Specs
| Cathode | Can be lithium manganese or NMC |
|---|---|
| Anode | Lithium Titanate (Li2Ti2O3) |
| Cell Voltage | 2.4V |
| Applications | Solar-powered street lighting, electric powertrain, and UPS |
| Cycle Life | Between 3.000 and 7.000 cycles |
With no graphite as the anode, the risk of SEI film formation and lithium plating (two of lithium batteries’ main degradation mechanisms) is eliminated.
Therefore, this type of battery suffers less degradation (over time) than the rest of lithium batteries, lasting longer.
In addition, LTO batteries are much more resistant to low temperatures and high charge/discharge currents.
Thermal stability is also superior when compared to other lithium chemistries.
The major drawbacks are the much lower energy density (lower inherent voltage of 2.4V) and that this type of battery is quite expensive, given its many advantages and incredible lifespan.
Why Do Lithium Batteries Degrade?
Battery degradation is often presented as complicated and challenging to understand. Nevertheless, understanding battery degradation is a crucial step in developing cheaper batteries that can last longer while offering higher performance.
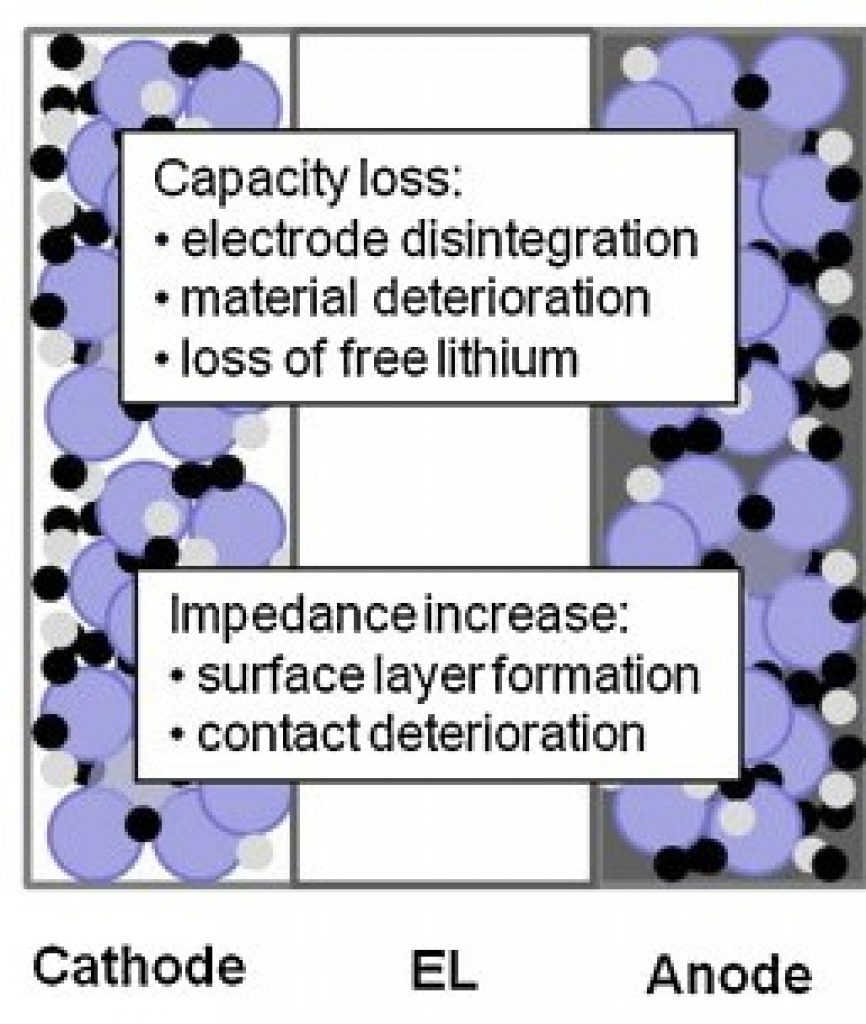
Like all batteries, the energy capacity of lithium batteries decreases with time and use while the internal resistance increases. This is a consequence of a wide range of degradation mechanisms, which can occur simultaneously and trigger further degradation mechanisms.
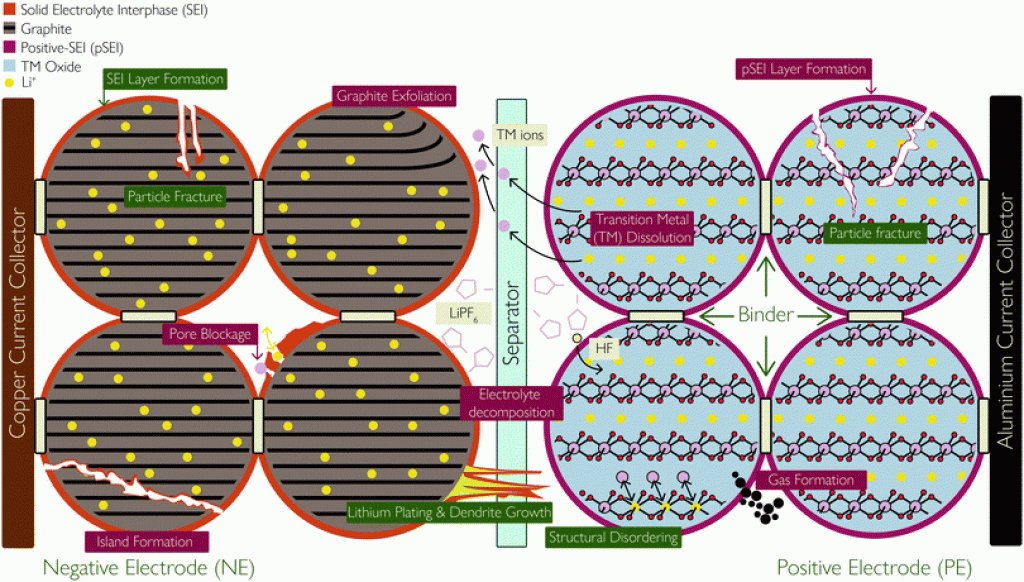
Source: “Lithium-ion battery degradation: what you need to know.”
In short, degradation mechanisms are related to the mechanical and chemical changes inside the battery cell.
Over time, or with improper use, the structure of the electrodes can be damaged, which increases the battery’s internal resistance. This leads to performance and capacity loss.
The Main “Stress” Factors That Can Lead To Degradation
- The formation of resistive layers (solid electrolyte interphase, or SEI) on the electrode’s surfaces;
- Lithium plating;
- Cracking of the SEI layer and damage to the electrode’s structure;
- thermal decomposition of the electrolyte.
The state of charge (especially a high one) also accelerates degradation. It can lead to irreversible side reactions, which use the materials that participated in the reactions that converted energy within the battery (the “active materials”).
With the loss of active material, the capacity and specific power decrease.
Similarly, when the lithium battery operates at a high current, the likelihood of failure increases due to mechanical stress and lithium plating during charge.
Finally, many factors can lead to the loss of electrolytes, increasing the battery’s internal resistance, which drastically impacts the battery’s performance. Factors that lead to increased internal resistance include SEI formation (Solid electrolyte interphase), high voltages, lithium plating, and high/low temperatures.
Source
Factors that affect lithium batteries
- The C-rate Of Charge And Discharge: the amount of charge/discharge current relative to batteries rated capacity.
- Storage Temperature: during charging, discharging, and storage.
- Depth Of Discharge: the extent to which you discharge your battery during each cycle.
- State Of Charge: how much capacity your battery has at a given point, relative to its total capacity.
- Improper Charging: overcurrent, overvoltage, overheating, etc.
Final Thoughts
Lithium-ion batteries (or simply LIB) have proven to be very reliable energy storage systems for stationary and mobile applications.
There are several different types of lithium-ion batteries. Each one offers advantages and disadvantages.
Depending on their cathode and anode material, they can offer a higher energy density, longer cycle life, higher specific power, etc.
Some materials are more stable than others, lasting longer and offering fewer safety risks. However, these batteries usually present a lower specific power and lower energy density.
Therefore, it is crucial to select the correct type of lithium battery for a specific application. In addition, good use practices and maintenance can help prolong the lithium battery cycle life.









Hey Ana,
thanks for this insightful report! 🙂
Regarding the Lithium Battery Cycle Life Chart,I am just wondering where did you get the figures from,
as I really want to use them in my Master Thesis.
I would highly appriciate your help,
and many regards,
Valentin
Hi Valentin,
Thanks for the compliment! We really appreciate your comment 🙂
Regarding your question: As you can see, I didn’t write specific numbers for each battery, I used a relatively wide range for their life cycle.
And that’s because I didn’t use one single source to find the data I used to build the chart. I gathered information from several sources/studies. As you know, different sources/studies will show you different information regarding cycle life (while one study can show that a NMC battery performs 1500 cycles, another one can say it performs 2000 cycles, for example). That’s normal, because cycle life depends on several factors (internal and external), so these numbers can vary greatly.
With that said, I’ll leave you a few links that I used while doing research for the article. Hope it helps!
Types of lithium batteries
NMC battery
Li-ion battery degradation
LCO degradation
LTO info
Lithium batteries
I work with a leasing company that is looking at financing a $3MM 1.6MM/5.57 MWh Lithium Ion BESS and am trying to understand what the useful life of the system might be in terms of ‘how many years’.
What data input would i need for rough estimate purposes? Ion type, average cycles/time unit? I did find some article that generalized a 10-15 years life span for lithium-ion batteries used for ‘behind the meter’ time shift purpose. Even if this is a good ‘ballpark’, does it assume each battery module lasts so long, or is there some understood failure rate that’s considered part of ‘proper maintenance’ of such systems. Unfortunately, until we get into this deeper, I don’t have anything further on the technology. The manufacturer’s (LG Chem) website is incredibly vague on the technology.
We need to offer our customer a 6-8 year financing term, which for tax purposes, cant’ exceed 80% of expected life, expectation being that there would be some value to system at end of lease in event of return.
Any thoughts?
Chris