Did you know that battery storage temperature can significantly impact your battery’s aging rate and overall performance?
Although batteries are highly present in our day-to-day lives, most people don’t know how to store them properly.
For this reason, a fair amount of batteries end up being placed/stored at the wrong temperatures – either too hot or too cold. And like most electrical devices, batteries don’t perform well at extreme temperatures.
Therefore, storing your battery under extreme conditions can significantly influence its lifespan and efficiency. Also, specific battery chemistries are more susceptible to these temperature-related problems than others.
In this article, we’ll discuss the correct (or most recommended) battery storage temperature and how batteries are affected by temperature. Additionally, we’ll tackle a few relevant questions, such as:
- At what temperature should you store a battery?
- What happens if you store a battery at the wrong temperature?
- How do you store batteries long-term?
So without further ado, let’s look at the answer to these questions, so your battery never again suffers from issues caused by the wrong battery storage temperature.
Table of Contents
At What Temperature Should You Store A Battery?
Batteries come in different shapes, sizes and chemistries. Therefore, it shouldn’t come as a surprise that different types of batteries have different appropriate storage temperatures.
In a broader sense, the recommended battery storage temperature is around 15ºC (59ºF). However, slight variations — ranging from 5ºC (41ºF) to 20ºC (68ºF) — are perfectly safe.
However, extreme temperatures — below -5ºC (23ºF) and over 35ºC (95ºF) — will most likely lead to problems (especially for lead-acid batteries) such as:
- Shortening of battery life
- Decrease of usable capacity
- Degradation of the battery’s components
- Lithium plating in lithium batteries
- Sulfation in lead-acid batteries
A Quick Review On Battery Related Terms
Before we deepen our discussion, here’s a review of a few basic concepts that should help you better understand what’s to come in the article.
Voltage: you can think of voltage as the pressure that pushes the electrons through a circuit.
Open circuit voltage: is the voltage between the battery’s terminals when the battery is not connected to a load (zero current being drawn or delivered).
Self-discharge: the chemical reactions inside the battery’s cell occur without any connection between the electrodes or an external circuit. This phenomenon reduces the battery’s stored capacity even when not in use.
Electrolyte: the electrolyte sits between the anode and the cathode and allows ions to move between these electrodes; thus, chemical energy is converted into electric energy.
State of charge (SoC): expresses the present capacity of the battery relative to its total capacity.
Can You Store A Battery In A Cold Place?
Yes, but it does depend on how cold the place is — storing your battery at freezing temperatures for an extended period (over 3 months) will most likely harm your battery.
Why? Because self-discharge rate and open-circuit voltage vary with ambient temperature.
Colder temperatures make the battery’s electrolyte less conductive; therefore, the open-circuit voltage decreases. This is undesirable because if a battery’s voltage drops below a certain point, the battery can suffer irreversible damage due to material degradation.
On the other hand, the self-discharge rate decreases in colder temperatures, which is a good thing.
In short, you can store a battery in a cold place, but only up to a point.
Most Manufacturers Recommend
- -5ºC (23ºF) and 35ºC (95ºF) for lithium batteries
- 0ºC (32ºf) and 30ºC (86ºF) for lead-acid batteries
For Periods Longer Than 3 Months
- 0ºC (32ºF) to 25ºC (77ºF) for lithium batteries
- 10ºC (50ºF) to 20ºC (68ºF) for lead-acid batteries
Aiming to avoid problems related to cold temperatures — especially in countries where the winter can be harsh — manufacturers developed batteries more resistant to low temperatures. These batteries come with a mechanism that can use the battery’s stored energy to heat the battery if a dangerously low temperature is detected.
Can You Store A Battery In A Hot Place?
The same goes for hot places. You can store a battery in a “hot place,” but only up to a certain point — extremely hot temperatures are harmful to batteries and highly dangerous.
The Arrhenius Equation gives the temperature dependence of the rate of the chemical reactions. We won’t get into a detailed discussion of this equation. Still, a valuable piece of information here is that chemical reactions occur more rapidly at higher temperatures.
Therefore, a high storage temperature will increase the self-discharge rate of batteries, resulting in a rapid loss of the stored capacity. This is harmful to the battery because the state of charge (SoC) also dramatically influences battery life and performance.
In addition, lead-acid batteries suffer the “memory effect.” Therefore, they can permanently suffer a capacity loss due to material degradation when left at a low state of charge for an extended period.
Safety
Another problem with high temperatures is related to safety. Lithium batteries have a reputation for catching fire or even exploding when the internal temperature rises too high.
Essentially, you can store batteries in “hot places” as long as the ambient temperature doesn’t exceed 35ºC. At this temperature, your battery won’t suffer significant issues. However, they might experience minor efficiency and durability losses. So it is not recommended to store them in high temperatures for an extended period.
In Summary
Extreme temperatures are the great enemies of batteries. Storing your battery in cold places and hot places will harm your battery’s durability, capacity, efficiency, and performance.
Therefore, avoid storing your battery at extreme temperatures below 0ºC (32ºF) or over 30ºC (86ºF), as they might harm your battery’s health.
What Happens If You Store A Battery In The Wrong Temperature?
To fully comprehend the effects of storage temperature on batteries, you must first understand how batteries work.
How Rechargeable Batteries Work
Rechargeable batteries, like Lead-acid and Lithium-ion, work according to the same principle: reversible reduction and oxidation reactions.
Inside their cells, there are three main components:
- Anode, the negative electrode
- Cathode, the positive electrode
- An electrolyte is a liquid (or gel) that contains ions and can be decomposed by electrolysis. The electrodes are immersed in the electrolyte.
When discharging, chemical reactions occur inside the cells, converting the stored chemical energy into electrical energy. When charging, the opposite reactions occur, so electrical energy is stored as chemical energy.
In Summary
Electricity is produced by the reactions that happen between the battery’s components.
Below is an illustration of how Lithium-ion batteries work:
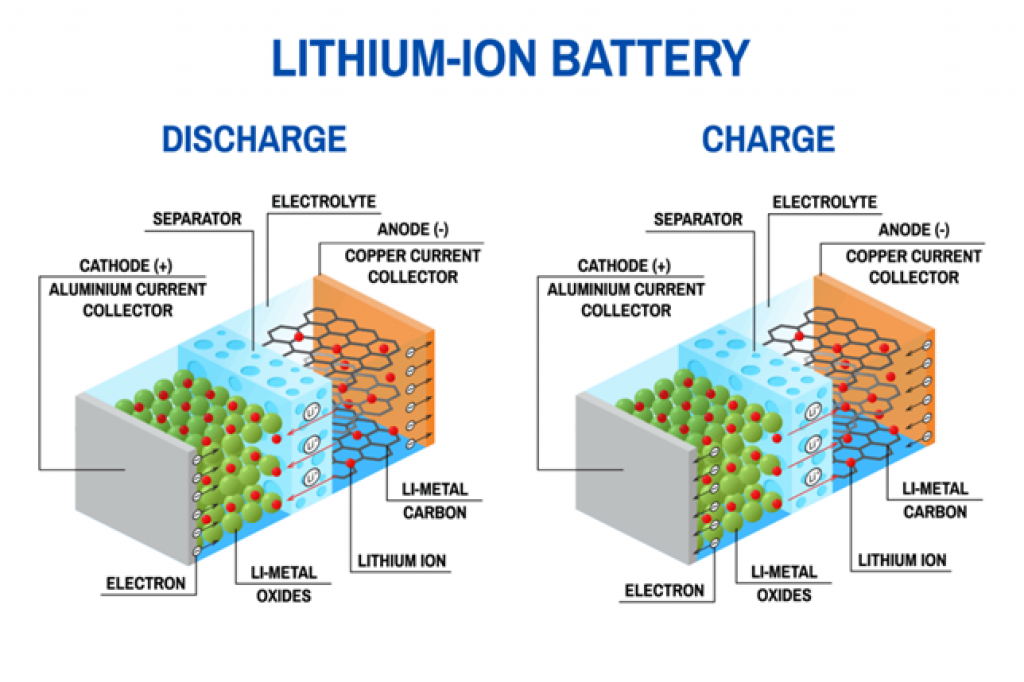
Source: borregaard.com
Lead-acid batteries work pretty much the same way, but the materials used are different:
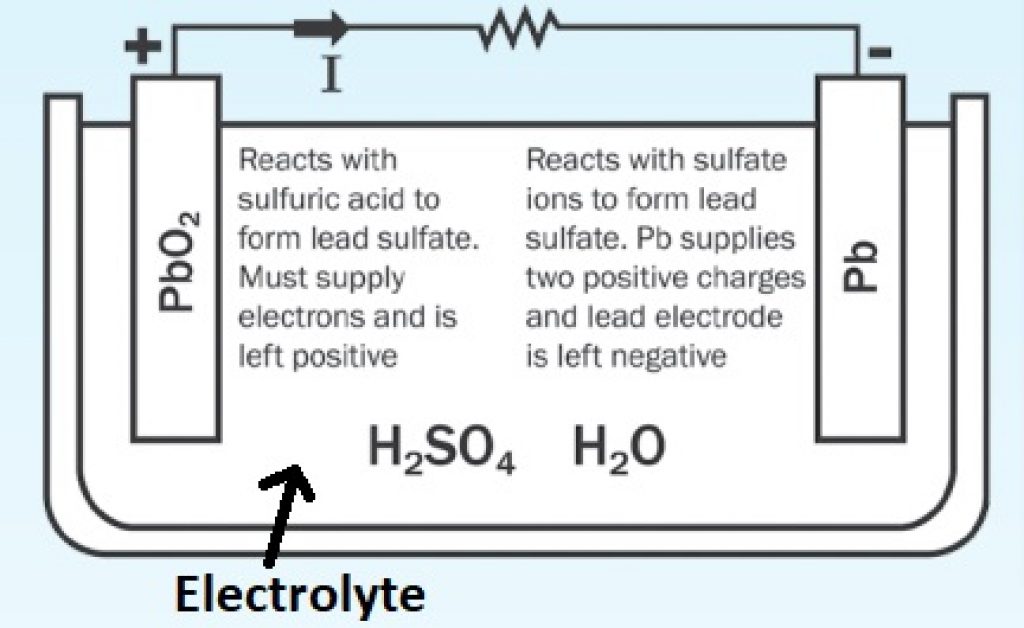
Source: power-sonic.com
Notice that, in both cases, the electrolyte is key to energy production. It serves as a medium for the ions to move between electrodes, allowing reduction and oxidation reactions.
This information is key to understanding how ambient temperature affects batteries.
What Happens If You Store A Battery In A Cold Environment?
Did you ever notice that your phone battery is drained much quicker during a cold day when temperatures are below 5ºC (41ºF)?
This happens because, in cold temperatures, the battery’s electrolyte becomes less conductive, which leads to an increase in the battery’s internal resistance.
Therefore, the voltage — that pressure that pushes electrons through a circuit — decreases, along with your battery’s power output (since power is the product of voltage and current).
However, when the battery is not being used, no electrons are pushed through a circuit.
Nonetheless, if you measure the voltage between the battery terminals, you’ll read the open-circuit voltage. This open-circuit voltage is the potential difference between the terminals, and it’s associated with the battery’s internal resistance.
The potential difference (the open-circuit voltage) decreases if the internal resistance increases. This can be harmful to the battery because the open circuit voltage is proportional to the battery’s state of charge.
In turn, storing your battery at extreme states of charge — either too low or too high — may lead to degradation of materials or loss of battery efficiency.
Think of it this way: if a lithium battery is stored at a high state of charge, too many lithium atoms will be ready to let go of their valence electrons (an extremely favorable oxidation reaction). Therefore, the battery will be in a stressed state.
Conversely, if you store it at a state of charge of around 40% or 50%, the chemical reactions will be at equilibrium so that the battery won’t be stressed.
A stressed state can lead to side reactions resulting in the formation of unwanted products that can lead to irreversible battery damage.
In Summary
Cold storage temperatures make the electrolyte less conductive, which increases internal resistance — the increase of internal resistance results in the decrease of open-circuit voltage.
The battery’s voltage is proportional to its state of charge — so the lower the voltage, the lower the state of charge. And when left at a low state of charge for too long, batteries can suffer irreversible damage.
Additionally, extremely low temperatures can lead to extremely low voltages (or even no voltage, 0V), meaning that the battery is “dead” or “sleeping.” If this happens, specific chargers with a “recovery” or “boost” function are necessary to revive the battery.
Depending on the battery chemistry, cold storage temperatures cause degradation of the battery’s components. The damage is often irreversible in such cases, rendering the battery unusable.
“Surely hot temperatures make the electrolyte more conductive, and as a result, more power is produced given the lower internal resistance = higher voltage?”
While the above assumption is reasonable, it isn’t accurate
What Happens If You Store A Battery In A Hot Environment?
Recall that we can calculate reaction rates using the Arrhenius Equation? We learn that chemical reactions occur more rapidly at higher temperatures from this equation.
What Does This Mean?
Here’s what happens: the high ambient temperature will increase the battery’s internal temperature, making its electrolyte more conductive and increasing the kinetic energy of particles contained in the battery’s cells.
With this, the frequency of particle collisions increases, and more collisions mean more reactions are happening.
Although there are many different ways to speed up a chemical reaction, the best one is probably increasing the system’s temperature.
But more reactions happening is a good thing, right? Doesn’t it mean that the battery is producing more energy? Not really.
Why?
Firstly, we’re discussing battery storage temperature. When a battery is stored, the desired scenario is that the chemical reactions occurring within the cells are kept to a minimum. This way, the battery won’t suffer any charge losses or material degradation, leading to permanent damage.
Secondly, the increase in collision frequency doesn’t mean that the right reactions will occur.
The chances of undesired side reactions occurring also increase. This can result in the permanent formation of undesired products.
The consequence of the formation of these byproducts is the irreversible decrease of battery capacity since there is less material to participate in the “desired reactions.”
Another possible outcome is that these byproducts can rupture the cell’s separator, resulting in a short-circuiting battery (direct contact of electrodes).
This permanently damages the battery cells and can result in a fire or explosion.
In Summary
Extremely hot battery storage temperatures — above 35ºC (95ºF) — increase the battery’s self-discharge rate, reducing its voltage and state of charge. It can also degrade the battery’s components, permanently damaging the battery or considerably speeding up its aging process.
Thus, to avoid shortening your battery’s life and minimizing its performance, you should store/ use your battery at hot temperatures.
Below is a temperature x self-discharge curve from the Canbat cold weather LiFePO4 battery’s user manual:
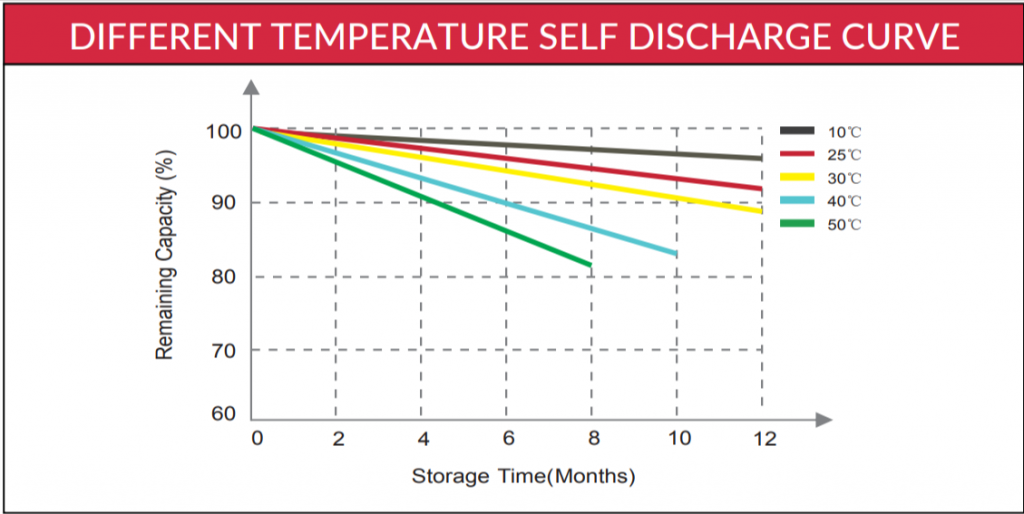
Notice that the remaining capacity (and the storage time) decreases for higher temperatures. That’s because the self-discharge rate increases with temperature, reducing the battery’s stored capacity.
How Do You Store Batteries Long-Term?
If you need to store your batteries for an extended period, there are a few measures you can take to maintain your battery correctly:
Avoid Extreme Temperatures
As extensively discussed in this article, the wrong battery storage temperature is the battery’s number one enemy. It considerably speeds up the aging process of your battery.
Therefore, always store your batteries within the recommended storage temperature range. This way, you’ll maximize your battery’s durability and overall performance.
You can find the most appropriate storage temperature for your battery in the user manual/datasheet provided by the battery’s manufacturer.
Monitor Voltage/State Of Charge
Even when stored within the recommended storage temperature range, batteries will inevitably self-discharge.
When batteries self-discharge, their voltage drops (as does their state of charge). If a battery stays at a low voltage for an extended period, it can go into a “sleeping mode,” which can harm the battery and shorten its lifespan.
To avoid this problem, always check your battery’s open-circuit voltage from time to time when not using it for an extended period. You can measure your battery’s voltage using a multimeter.
Once you know your battery’s voltage, you can use a state of charge chart to find out the SoC of your battery.
If the voltage/state of charge is too low for your battery — for instance, if your 12V LiFePO4 battery is showing a 12.8V (10% SoC) or even 10.8V (1% SoC) — then you should recharge your battery a bit (just up to 50% SoC, 13.1V) to avoid any age-related issues.
The same goes for lead-acid — because they have a high self-discharge rate, you should recharge your stored lead-acid batteries periodically to avoid permanently damaging them.
Control Humidity
Storing your batteries in a place with high humidity can cause condensation and corrosion within the battery.
You should avoid condensation and corrosion as they will most likely cause permanent damage to your battery, reducing its service life and capacity to store energy.
Therefore, manufacturers highly recommend storing batteries in dry places.
Store The Battery In A Safe Environment
If your battery, for some reason, falls from a certain height, or if an object falls on your battery, the impact can rupture the cells causing a spill/leakage of battery chemicals.
This not only damages the battery but can also be very dangerous.
Lead-acid batteries, for example, can leak sulfuric acid if you damage their structure. Sulfuric acid is highly corrosive, cause severe burns to the skin, and can violently react with water, releasing heat.
Final Thoughts
Battery storage temperature can either be a great villain or a great ally to your battery’s health.
Storing your battery at just the right temperature can help minimize self-discharge and loss of stored capacity. It also avoids material degradation, shortening of battery life, and efficiency losses.
However, if you store your battery at extreme temperatures for an extended period, it will probably suffer irreversible damage. Hot temperatures are even more concerning as they not only damage the battery but can also pose serious safety risks, like fire or explosion.
If you don’t plan on using your battery for an extended period, the best thing you can do to preserve your battery’s health is to store it in a place with the right temperature and humidity.
Recommended battery storage temperature may vary according to the battery’s chemistry, so checking the user manual is the best way to determine the optimal storage temperature for your battery.
As a rule of thumb, optimal battery storage temperature is between 10ºC (50ºF) and 20ºC (68ºC).
Acceptable storage temperatures — as recommended by many battery manufacturers — range from -5ºC (23ºF) and 35ºC (95ºF) for lithium batteries and 0ºC (32ºf) and 30ºC (86ºF) for lead-acid batteries.
For periods longer than 3 months, the recommended storage temperature ranges from 0ºC (32ºF) to 25ºC (77ºF) for lithium batteries and 10ºC (50ºF) to 20ºC (68ºF) for lead-acid batteries.
However, you should never store your battery in places that can reach extreme temperatures – below —5ºC (23ºF) and over 50ºC (122ºF) — as doing so will definitely harm your battery and may even result in a fire or explosion.
At the proper storage temperature range, properly storing your batteries will extend their shelf life and reduce safety hazards considerably.

