How do hydrogen fuel cells work? Well, fuel cells are devices that generate electricity through an electrochemical reaction instead of combustion.
Experts in the energy sector have been improving this technology in the search for emission-free energy source alternatives to the traditional combustion processes.
Hydrogen fuel cells are one of the promising technologies for the mid and long term since they allow storing hydrogen as an energy source.
Hydrogen is not readily available in the environment, it is always part of another substance. This is why a chemical conversion process must be carried away from sources like oil or water to obtain this new value fuel.
However, this article will not focus on the conversion processes to obtain hydrogen, but rather on the application of this element as a source of energy in fuel cells.
You will learn how do hydrogen fuel cells work, their benefits and disadvantages, and finally what you can expect from them in the next couple of years.
Table of Contents
How Do Hydrogen Fuel Cells Work?
What Are Fuel Cells?
Fuel cells are electrochemical devices that convert the chemical energy of a fuel and an oxidizing agent into electricity.
Redox reactions are responsible for this. This chemical reaction changes the oxidation states of atoms.
Fuel cells and batteries are similar as they both generate electricity.
However, a battery stores energy generally in a lithium or sulfuric acid solution, while fuel cells use an external source such as hydrogen allowing it to continue operating as long as fuel is available.
All fuel cells have similar basic configurations, an electrolyte, and two electrodes, but there are different types of fuel cells. This is based on what kind of electrolyte they use to produce the chemical reaction.
The most popular fuel cell type is the Polymer Electrolyte Membrane (PEM), which uses perfluorosulfonic acid as its electrolyte membrane.
This type of fuel cell is commonly used to make hydrogen fuel cells.
How Do They Work?
A fuel cell consists of two electrodes, an anode, and a cathode, separated by an electrolyte membrane. A fuel (hydrogen in this case), is fed to the anode, and air is fed to the cathode.
In a hydrogen fuel cell, a catalyst at the anode separates hydrogen molecules into protons and electrons, which take different roads to the cathode.
The electrons go through an external circuit, creating a flow of electrical energy while the protons migrate through the electrolyte to the cathode, where they join with oxygen and the electrons to produce water vapor and heat as a result of the process.
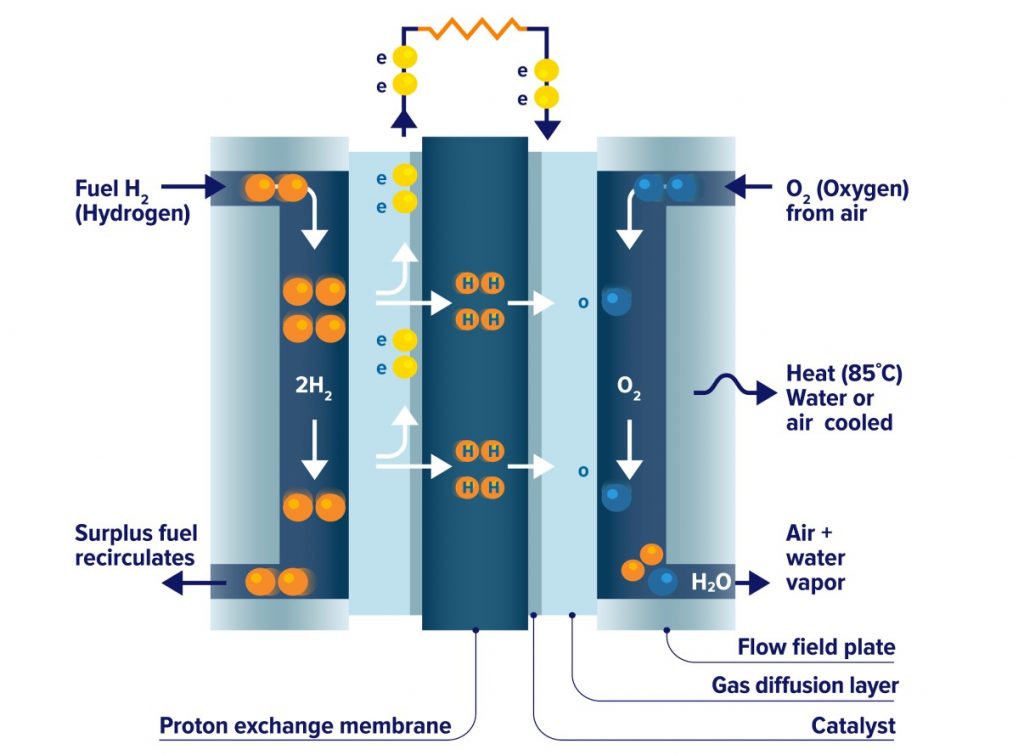
Figure 2. Functioning Of A Hydrogen Fuel Cell. Source: Canadian Hydrogen And Fuel Cell Association
How Long Do Hydrogen Fuel Cells Last?
These fuel cells will last as long as hydrogen and oxygen are supplied to them.
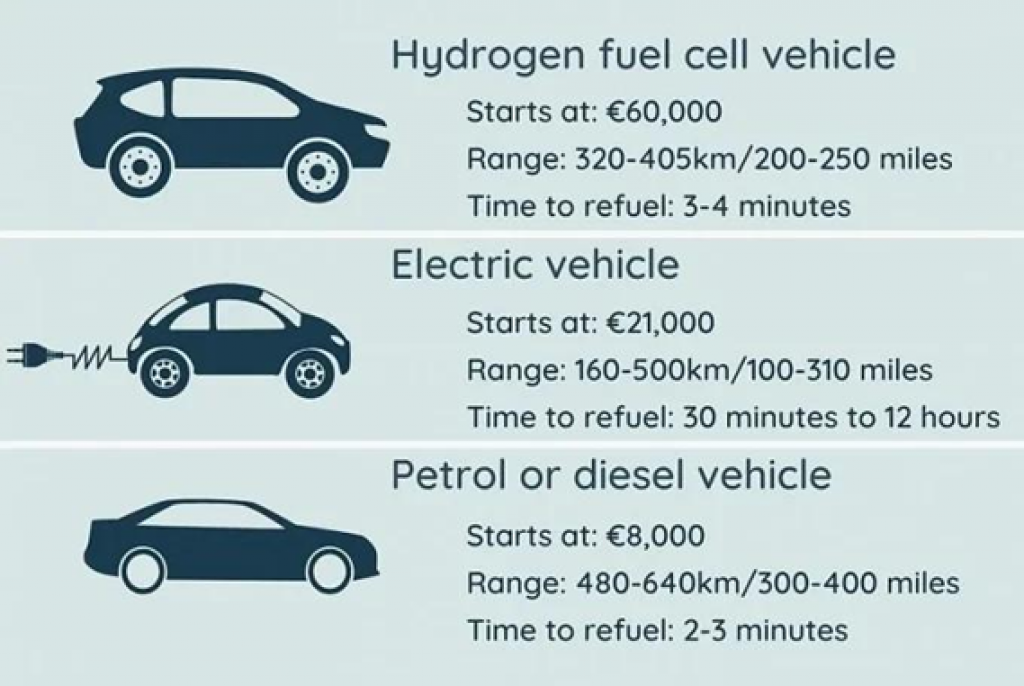
In vehicles, the hydrogen fuel cells stacks would last 320-405Km (200-250 miles) per refuel. These stacks are designed to last throughout the lifetime of the vehicle – 150,000–200,000 miles.
Fuel cell stacks are made up of multiple fuel cells layered together. Depending on the application, the fuel cell stack may contain hundreds of single cells put together.
At the end of its lifespan, the fuel cell stacks are dismantled and the materials recycled.
In portable applications, some generators based on hydrogen fuel cells can be combined with batteries, solar panels, or small wind turbines to improve their performance.
It is claimed that they can have a lifespan of 5,000 hours.
How Much Electricity Does A Hydrogen Fuel Cell Produce?
According to the US Department of Energy, a single fuel cell can only produce around 0.5V to 1V.
As such, fuel cells must be piled together in a stack or combined with other sources of energy in order to produce enough electricity to run appliances or vehicles.
Depending on how many fuel cells are stacked together, these kinds of cells can generate between 1 kW to 100 kW of power.
An example of how much energy these cells can generate in big-scale applications can be found at Hanwha Energy’s hydrogen fuel cell power plant at the Deasan industrial complex, located in South Korea.
The plant contains 114 fuel cells and can generate up to 400,000 MWh of electricity per year.
This is enough electricity to power 160,000 homes, making it the world’s largest industrial power plant that uses hydrogen fuel cells.
Another example, now in portable applications, is a fuel cell-based generator that can be used in off-grid applications.
The generator, named UP400, is claimed by Estonia-based hydrogen fuel cell specialist “PowerUP Energy Technologies” to have a minimum lifespan of 5,000 working hours and a power output of 400 W.
It operates at temperatures ranging from -20 to 52°C. The device has a weight of 10kg (22 pounds) and is based on PEM fuel cell technology to work.
What Are The Pros and Cons Of Hydrogen Fuel Cells?
Fuel Cell Advantages
Fuel cells have several benefits over conventional combustion-based technologies currently used in many appliances and vehicles.
Here are some of these advantages:
Higher Efficiencies Than Combustion Engines
Fuel cells can operate at higher efficiencies than combustion engines and can convert the chemical energy contained in the fuel directly into electrical energy with efficiencies capable of exceeding 60%.
Low Greenhouse Emissions
Hydrogen fuel cells have zero greenhouse gas emissions compared to combustion engines.
These fuel cells do not generate pollutants that contaminate air or that cause health diseases. This is because hydrogen fuel cells emit only water vapor and heat.
Quiet Operation and Low Maintenance Cost
These devices don’t produce vibration or noise during operation because they have few moving parts.
Thanks to this fact and its compact size, hydrogen fuel cells have low maintenance costs, longer lifetimes, and flexibility in installation and operation.
Quick Refuel and Response
Hydrogen fuel cell vehicles take just over 5 minutes to refuel their cells, making them much quicker to recharge than electrical vehicles.
Hydrogen fuel cells also count with rapid response to changes in electrical demand.
Fuel Cell Disadvantages
There are a number of issues related to the manufacturing of hydrogen fuel cells.
Here are some of them:
Expensive Production
Hydrogen as a fuel for these cells is extremely expensive to produce, store, and deliver. This makes them less viable to mass produce in vehicles and bigger appliances.
Additionally, there aren’t many places to refuel these cells throughout the world.
Production Efficiency Losses
Energy loss also occurs from the hydrogen production process.
There is a 20% loss in energy during electrolysis – when hydrogen is separated from other substances.
A further 13% is lost when hydrogen is compressed into a fuel.
This means that to obtain hydrogen nearly 30% of energy is lost.
Are Hydrogen Fuel Cells The Future?
Hydrogen fuel cells have multiple applications such as backup power, portable power, distributed generation, transportation, and vehicles.
These fuel cells may be more common in our cars, homes, and jobs in the near future.
According to many experts, we may soon find ourselves using fuel cells to generate electrical power for all sorts of objects we use every day.
The worldwide market for hydrogen fuel cells was valued at 1,686 million USD in 2020 and is expected to reach 4,476.2 million USD by the end of 2026, growing at a compound annual growth rate (CAGR) of 14.8% during the 2021-2026 period.
The growth is attributable to the rising demand for clean energy generation in developed regions.
However, right now it is still too soon to see the widespread development of big scale projects due to high capital costs.
Fuel cells cannot yet compete economically with traditional energy sources, though technical advances are being made to address this issue.
Nevertheless, the pandemic has represented a major setback in the development of fuel cell plants as with other clean energy projects.
The COVID-19 crisis has significantly affected the addition of renewable power capacity, which has also affected fuel cell developments.
Taking the IEA (International Energy Agency) as a reference, the renewable energy market declined by 13% when comparing the net expansion of renewable capacity from 2019 to 2020.

Final Thoughts
The role and use of fuel cells in stationary and portable applications, such as vehicles and portable devices could be significant in the near future, especially if opportunities for integrated systems are considered.
These fuel cells have proven to be a resource of clean energy with a low cost of maintenance and quiet operation that can work for long periods of time as long as hydrogen is supplied.
These cells surpass the efficiencies of combustion engines and recharge speeds of electrical vehicles.
However, the economic constraints to sustain these cells are an important limitation to expand market shares.
This means that we will have to wait for a few more years until economies of scale do their part and manufacturing costs for hydrogen fuel cells become more competitive.

