When choosing the correct lead-acid battery for your needs, there are only two worth considering — AGM vs. Gel.
These two battery types are the most common sealed lead-acid (SLA) batteries. When these technologies include a one-way blow-off valve, they are referred to as valve-regulated lead-acid (VRLA) batteries.
AGM and Gel batteries were designed almost twenty years apart, with AGM being the most recent. Despite the difference in time span, the two batteries have some elements in common — both have lead plates (same chemistry) and the ability to deep discharge.
However, their design and overall performance hold a few defining differences. The main one is the type of membrane that separates the plates and contains the electrolyte.
The AGM battery has an “Absorbent Glass Mat” between the lead plates that acts as a sponge to hold the electrolyte between the plates. On the other hand, silica is added to the electrolyte in Gel batteries to form a thick mixture (gel) that keeps the electrolyte in between the lead plates.
For this reason, both AGM and Gel are non-spillable since the battery is not flooded with the electrolyte (as it is in flooded lead-acid batteries). The electrolyte (acid) is contained in the separator.
But if both AGM and Gel batteries have their pros and cons, how do you know which one is right for you? In this article, we explain the chemistry of AGM and Gel batteries in detail.
Additionally, we take a look at the differences between AGM vs. Gel batteries to help you make an informed decision when deciding which is the best fit for your needs.
Table of Contents
Sealed Lead-Acid Batteries: AGM And Gel
Before we move on to the more detailed comparison of AGM vs. Gel batteries, let’s first review deep-cycle batteries.
There are two types of lead-acid batteries: starter batteries and deep-cycle batteries.
Starter Battery
A starter lead-acid battery’s primary purpose is to start machines by providing a high surge current (100+ Amps) for a very brief period (a few seconds or less).
Once the machine turns on, it no longer draws current from the starter battery.
Therefore, starter batteries are designed to deliver a high current for a short period of time.

Source: racshop.co.uk
This type of battery is used primarily in vehicles. It provides enough energy to create a spark that will start the vehicle’s internal combustion engine.
Deep Cycle Battery
On the other hand, lead-acid deep cycle batteries are built to provide a relatively low current (less than 20 amps) for an extended period (several hours).
For this reason, they can power appliances and devices that draw a low current for quite some time.
The term “deep cycle” comes from the fact that you can discharge these batteries 50% to 80% of their total capacity.
We use deep cycle batteries in solar battery systems. They are the most appropriate for this application as they can withstand delivering a continuous current for an extended period without suffering any immediate damage.
Structural Difference Between Starter Batteries And Deep Cycle
What makes it possible for one battery to deliver a high burst of current for a short time and the other to a low amount of current for an extended period?
The answer lies in the thickness of the lead plates.
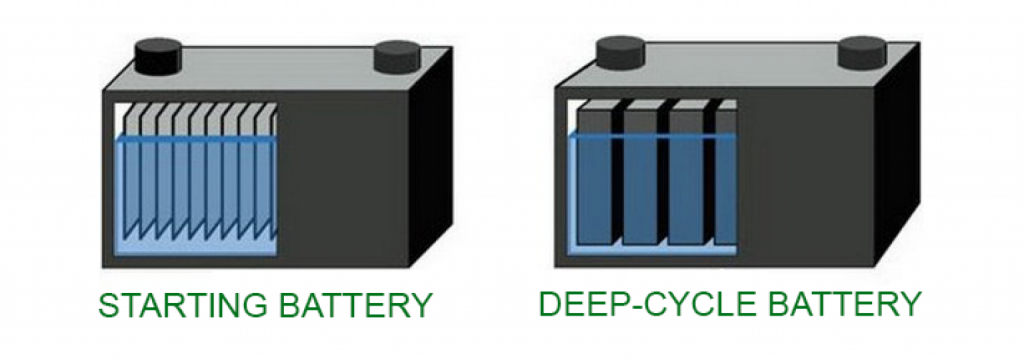
Source: everexceed.com
Deep cycle batteries have much thicker lead plates than starter batteries. This difference in plate thickness makes the plates in the deep cycle battery more resistant and less likely to buckle.
Therefore, they don’t suffer immediate damage when delivering current for a long time.
Lead is a soft metal, so if you used a starter battery as a deep cycle battery, the thin lead plates would most likely start to bend/lose their shape. This would impact the battery’s performance and ultimately reduce its service life.
Another factor that makes deep cycle batteries more resistant to a great depth of discharge is the type of separator that goes between the lead plates.
Two types of lead-acid batteries (AGM and Gel) were designed as part of an effort to increase durability and improve performance.
In this article, we discuss AGM vs. Gel batteries for deep cycle purposes.
Lead-Acid Batteries: AGM Vs. Gel
To understand how AGM batteries are different from Gel, you first need to understand how a lead-acid battery operates since this chemistry applies to AGM and Gel batteries.
Within the battery, there are several cells (the number of cells determines the battery’s voltage). 12V lead-acid batteries have 6 cells connected in series.
Main Components Of A Lead-Acid Cell
Within each cell, you’ll find:
- Lead plates (positive, PbO2(s) and negative, Pb(s))
- A separator between these plates
- Electrolyte to promote redox reactions
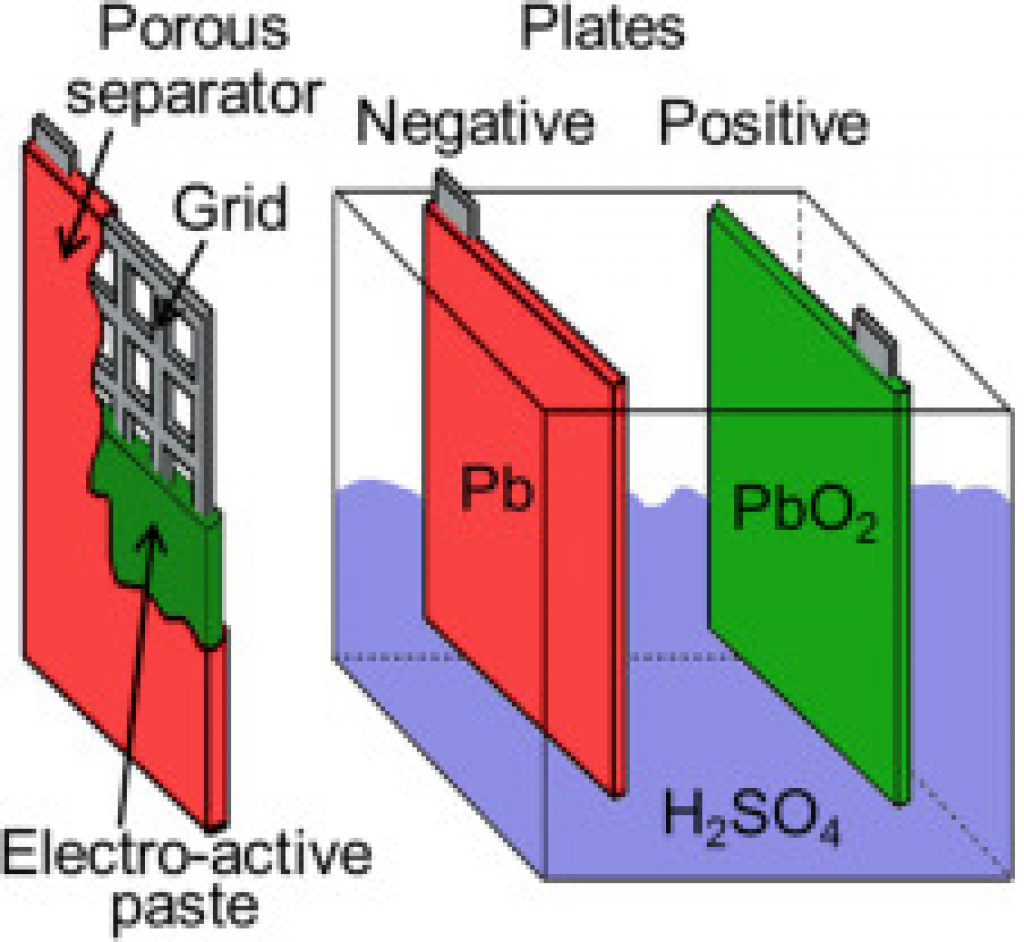
Source: “Aqueous batteries as grid-scale energy storage solutions.”
The lead plates are shaped as a grid to increase surface area. In addition, an electro-active paste is spread around the plates.
The material used as the separator depends on the type of lead-acid battery. In AGM batteries, the separator is a glass mat. In Gel batteries, silica is added to the electrolyte to form a gel that acts as the separator.
The electrolyte is a sulfuric acid (H2SO4) solution.
Chemical Reactions
Now that you’ve seen the main components of lead acid batteries let’s take a deeper look at their electrochemistry.
Like all rechargeable batteries, the lead acid battery has electrochemical cells where reversible chemical reactions occur.
When you charge the battery, electrical energy converts into chemical energy, conversely, during discharge, chemical energy converts into electrical energy.
What Are These Reactions That Convert Energy?
The positive plate is lead dioxide (PbO2) in lead-acid cells, and the negative plate is lead (Pb).
These plates are separated and react with the electrolyte (participating in reduction and oxidation reactions).
The electrolyte is an aqueous solution of sulfuric acid (H2SO4).
When you fully charge a lead acid battery, the sulfuric acid solution is at its highest concentration:
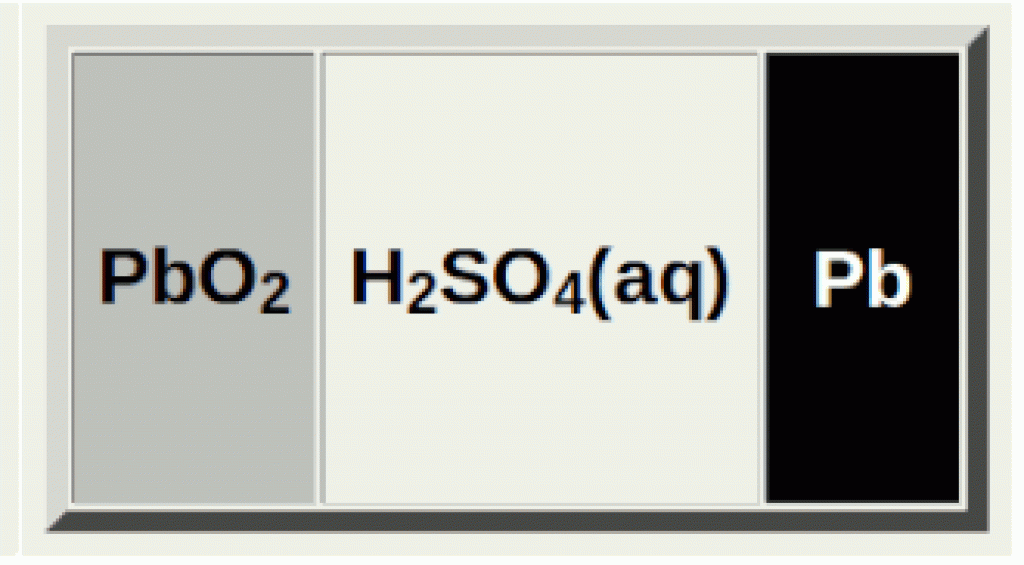
Pb(s), PbO2(s) and H2SO4(aq)
Once you connect the battery to a load, discharge occurs spontaneously. The “negative” plate suffers oxidation during discharge — the Pb reacts with the electrolyte to form PbSO4 (releasing electrons in the process).
Semi-reaction: Pb(s) + HSO−4(aq) → PbSO4(s) + H+(aq) + 2e−
Meanwhile, the “positive” plate suffers reduction: PbO2 reacts with the acid to form PbSO4(s) and water.
Semi-reaction: PbO2(s) + HSO−4(aq) + 3H+(aq) + 2e− → PbSO4(s) + 2H2O(l)
This process converts chemical energy stored in the cell into electrical energy, that can power devices.
Once you fully discharge the battery, the electrolyte is at its lowest concentration (very diluted), and both electrodes are mainly PbSO4.
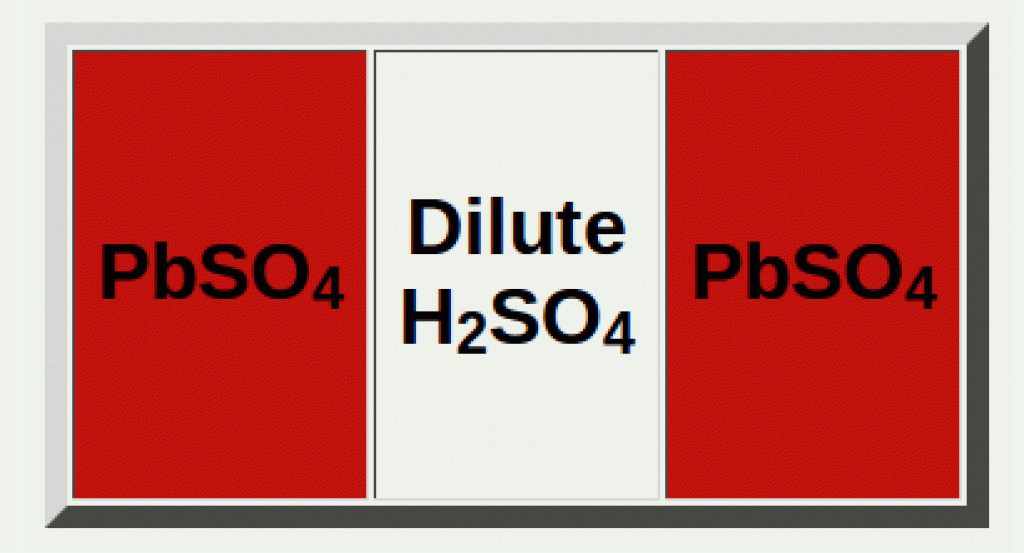
PbSO4(s) and diluted H2SO4(aq)
When you apply an external power source to the battery, the opposite reactions occur, converting electric energy into chemical energy. This way, the battery can perform many cycles.
Total reaction: Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
The potential difference of this electrochemical cell is +2.05V. That’s why a 12V battery has six cells connected in series, because 6 x 2.05V = 12.3V (nominal voltage of a 12V lead-acid battery).
Do Lead-Acid Batteries Need To Be Vented?
In the reactions shown previously, you’ll notice that, during discharge, 2H+ reacts with O2- ions to form water.
If there’s enough energy (an overcharge, for instance) for the electrolysis of water or if contact between these chemical species (H+ and O2-) is not facilitated, hydrogen and oxygen gases could form. When this happens, you need to vent the battery.
Not only is this bad for the battery (since it consumes the electrolyte), but it can be a highly flammable mixture.
In addition, overcharging could lead to the formation of hydrogen sulfide (H2S), which is easily detected by its strong odor of rotten eggs. This also indicates that side reactions are happening.
Flooded lead-acid batteries vent significantly more than SLA batteries (AGM and Gel). Even though SLA batteries aren’t entirely immune to venting, it is an incredibly rare occurrence.
This difference results from the technologies in each SLA, particularly in the separator used in each SLA battery.
For instance, gas bubbles in a flooded cell float to the top of the battery and are lost in the atmosphere. Meanwhile, in a sealed lead acid battery (AGM and Gel), the membrane that separates the lead plates allows gas transport through the separator.
Therefore, hydrogen or oxygen gas produced within the cell can freely pass through the separator to reduce or oxidize the opposing plate.
Because you don’t need to vent SLA batteries, you can view them as being maintenance-free. However, that doesn’t mean they don’t require a bit of battery maintenance to maximize performance and service life.
AGM Battery Chemistry Explained
An AGM battery is a type of lead-acid battery (a sealed lead acid or “SLA”), in which the separator used between the negative and positive lead plates is a very thin fiberglass mat called Absorbent Glass Mat.
This separator physically keeps the plates apart and absorbs the electrolyte due to the glass mat separator’s high capillarity.
Scheme Of An AGM Cell
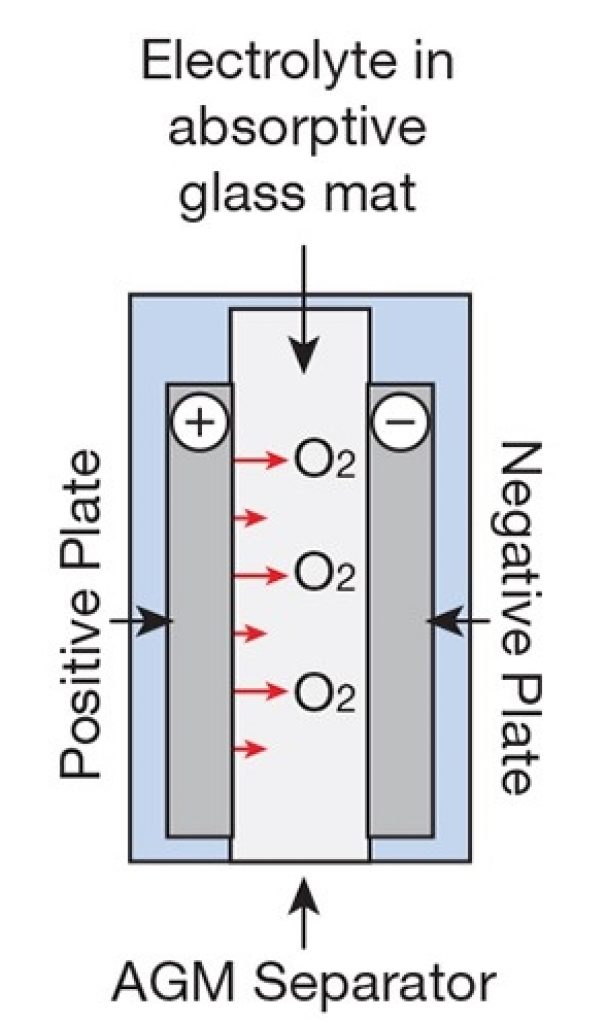
Source: Adapted from Home Power Magazine
This thin glass mat contains the electrolyte, giving this battery a few advantages over the flooded lead-acid.
The most important advantage is that this battery allows gas to freely pass through the separator (the glass mat soaked in the electrolyte).
Moreover, in an AGM cell, the electrolyte becomes one with the separator, making the cell mechanically robust. This allows the plate stack to be compressed together in the battery, which increases energy density (compared to flooded or gel).
This strong cell structure facilitates contact between the reacting chemical species (gas recombination is more efficient). Therefore, performance increases and reactions occur at a faster rate. In addition, the “tight” cell structure makes the battery more resistant to vibrations/impact.
The AGM technology also offers low internal resistance, which allows the quick delivery of high currents. For this reason, this technology is also commonly applied to starter batteries.
Ultimately, this lead acid battery is the safest because it retains water. The risk of hydrogen and oxygen formation is significantly reduced, leading to fewer accidents/risk of explosion, etc.
Finally, the mat prevents the vertical motion of the electrolyte within the battery. For this reason, you can store AGM batteries for longer without suffering significant damage/capacity loss.
Overall, the AGM technology maximizes the cycle life and performance of the battery.
Gel Battery Chemistry Explained
In a gel battery, silica is added to the electrolyte. This converts the liquid inside the cell into a semi-stiff paste (gel), providing many of the same advantages of the AGM.
For instance, the gel battery is spillproof because the gel mixture “immobilizes” the sulfuric acid. In addition, if there’s any damage to the battery case, like a puncture, etc., acid won’t easily leak from the cell.
Gas recombination is another advantage. The gel facilitates gas to flow through the cell to recombine, reducing safety risks and eliminating the need for venting.
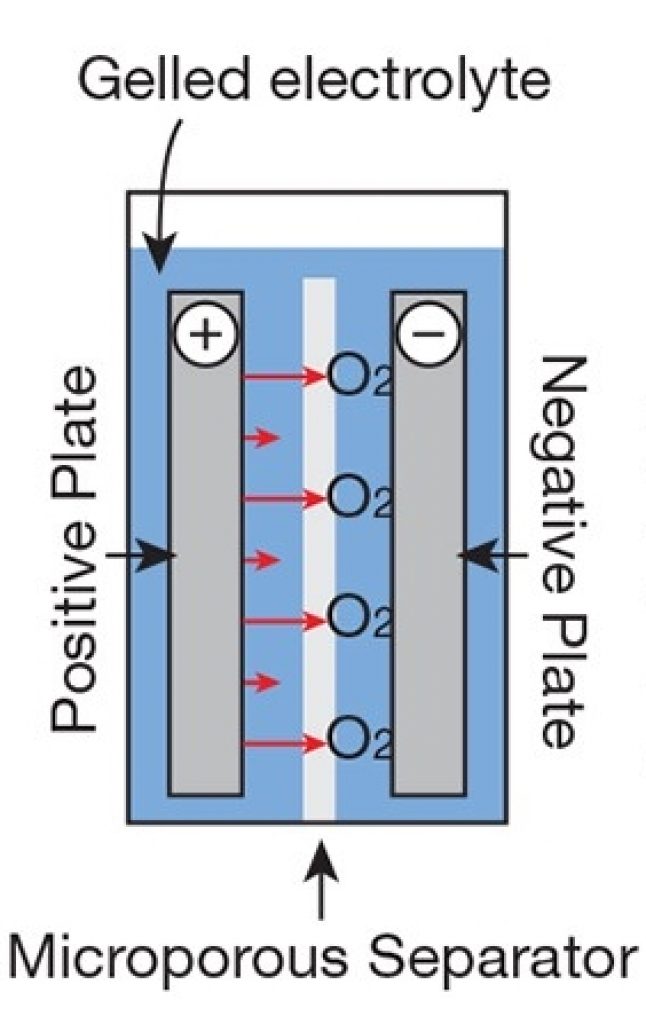
Source: Adapted from Home Power Magazine
Maintenance And Durability
Given the viscosity of the gel, this type of battery is less susceptible to evaporation, and it’s often used in situations that require little to no maintenance.
Additionally, the gel has a lower freezing point and higher boiling point than the liquid electrolytes used in flooded cells and AGMs, making them suitable for extreme conditions (especially high temperatures).
Old Design
Because gel batteries were developed in the 1970s, their design is a bit outdated, and there are currently very few engineering options left to improve them.
Disadvantages
What’s more, the gelled electrolyte offers significant disadvantages compared to AGM batteries.
Firstly, during charge and discharge, the gel tends to develop “pockets” (or cracks) with the current increase due to the gel’s high viscosity. These pockets prevent acid flow in the cell, resulting in battery capacity loss.
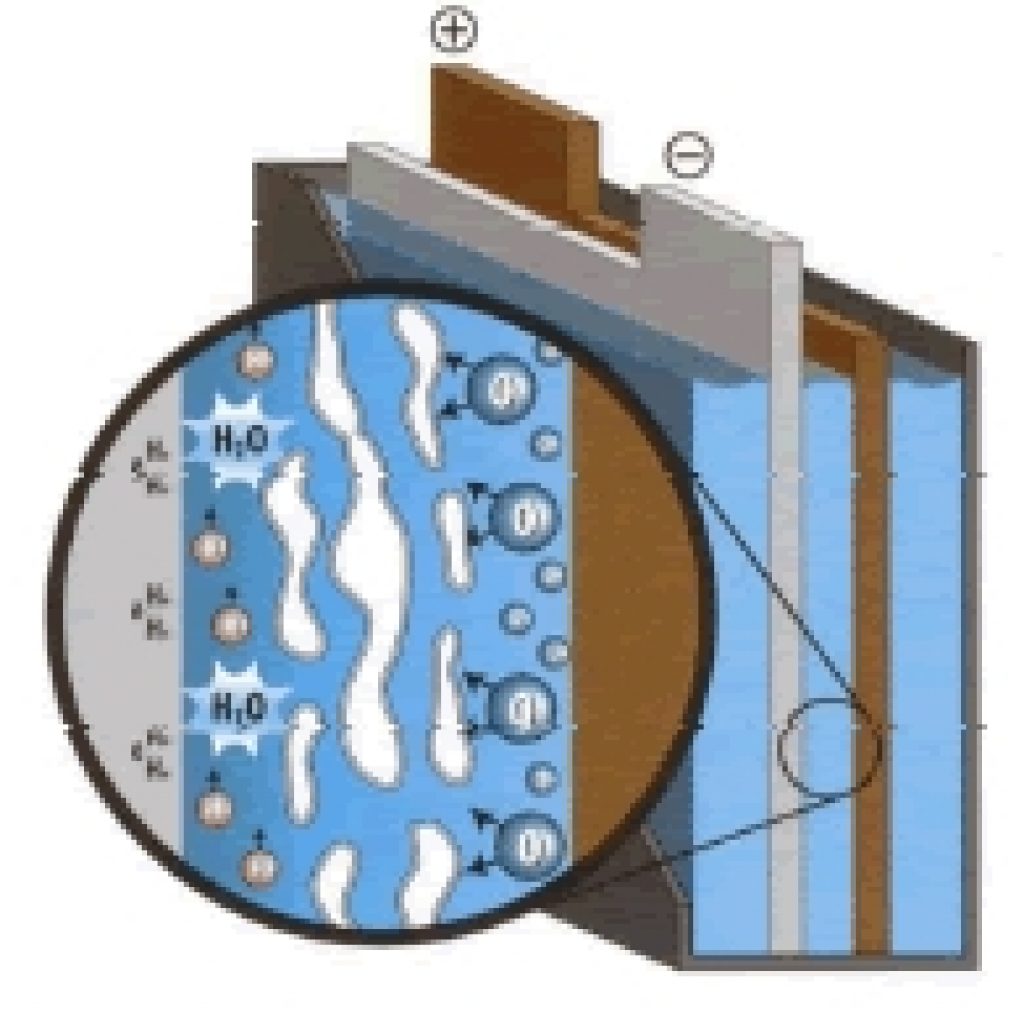
Source: batteryguys.com
Another disadvantage of the gel battery is that the gel prevents rapid motion of the ions in the electrolyte. This reduces carrier mobility and thus surges current capability.
For this reason, gel batteries are most commonly applied as deep cycle batteries, widely used in off-grid systems.
AGM Vs. Gel Batteries
While both offer many advantages (and a few disadvantages), one type might excel in a specific area and lack in another.
Common Traits
Before we mention the differences between AGM and Gel, let’s review their common traits:
- Maintenance free: watering for venting is not required because gas recombines within the cell to form water
- Both are spillproof since glass mat or gel contains the electrolyte
- Superior to flooded lead acid batteries in many aspects, including safety, cycle life, and resistance to vibration and extreme temperatures
- Lighter than flooded batteries
- Transportation/shipping is easier
- Possible to install it at any angle/orientation
Where AGM Shines
AGM batteries are generally more advanced than Gel batteries. Their design is more recent, providing more advantages and fewer disadvantages.
For example, AGM batteries have low internal resistance, which allows them to deliver high currents and function as starter and deep cycle batteries. Not only can this type of battery deliver high currents, but it can also receive high currents, which allows for fast charging.
In contrast, Gel batteries have higher internal resistance, limiting their surge current capability (the gel is quite viscous). Gel also takes much longer to charge (from 10h to 14h).
Although both batteries facilitate gas recombination within the cell and are therefore maintenance-free and safer than flooded cells, their performance and lifespan differ considerably.
Depth Of Discharge
Because gel batteries can’t deliver a high amount of current, they can usually endure greater depths of discharge than AGM.
Energy Density
In terms of energy density, AGM is best due to its compressed cell structure.
Discharge Rate
According to Peukert’s Law, the rate at which you discharge a lead-acid battery impacts the battery’s capacity. The greater the rate of discharge, the lower the capacity.
For example, consider a 12V 100Ah AGM battery. If you draw 20A from it, it will last longer (have more usable capacity) than if you discharge it at 80A.
Because Gel has a higher internal resistance, it is more affected by Peukert’s law.
Main Differences: AGM Vs. Gel
Finally, here’s a list summarizing the main differences between AGM and Gel:
| AGM | Gel |
|---|---|
| The electrolyte is contained within the glass mat separator | Silica is added to the electrolyte to turn it into a gel |
| Lower internal resistance | More resistant to high temperatures |
| Higher surge current capability | Gel’s viscosity prevents rapid motion on ions = can’t deliver a high surge current. |
| Lower self-discharge rate | Allow deeper levels of discharge without immediate damage (DoD up to 90%) |
| More resistance to vibration | The gel can be damaged (suffers cracks or “pockets” formation) with high amperage, reducing capacity. |
| Cheaper | A bit more expensive |
| Slightly shorter lifespan (550 cycles for 50% DoD) | Up to 1200 cycles (for 50% DoD) |
| Lighter | Does not allow for fast charging, taking up to 14h to fully charge |
| Good for sports vehicles | |
| Proper for power-hungry appliances |
Should You Buy An AGM Or Gel Battery?
Deciding between an AGM vs. Gel battery can be tricky, as both offer many advantages.
However, you’ll be able to find the type of SLA that best suits your needs after considering the following factors:
Factors You Should Consider
- What will you use it for?
- What’s your usual DoD?
- Are you using it to feed power-hungry devices?
- At which temperature will you charge it/store it?
- Will it be susceptible to vibration (in a motorcycle or jet ski)?
- What’s your budget?
- Is fast-charging one of your requirements?
Why Should You Buy An AGM Battery?
The fact that AGM has a low internal resistance means it can deliver a high current surge. For this reason, you can use an AGM battery as a starter battery. Meanwhile, Gel is more appropriate for off-grid systems (functioning as a deep cycle battery).
In addition, AGM battery allows fast charging, while Gel does not.
Another scenario in which AGM would be more suited is if you’re using it in sports vehicles, like a motorcycle or a jet ski, for instance. Why? Because AGM has a more robust cell pack and is, therefore, more resistant to vibration.
If you’re using your battery for a stationary solar system, both AGM and Gel will perform well. However, if you’re planning on using your battery system to feed power-hungry devices, you should choose an AGM battery.
Finally, although AGM batteries have a slightly shorter cycle life than gel batteries, they are less expensive. So if you’re on a budget, look for an AGM battery.
Why Should You Buy A Gel Battery?
Gel batteries can withstand deep discharge levels without suffering immediate damage. If you discharge a gel battery to 90% frequently, it will still perform around 700 cycles, more than an AGM battery frequently discharged at only 50% DoD.
Therefore, if you plan on discharging your battery for over 50% of its capacity, you should probably opt for a gel battery if you want to maximize your battery’s cycle life.
Lastly, gel batteries are more resistant to cold temperatures, so if you’re going to use it/store them in colder temperatures, the gel battery is the best option.
FAQ
Why might someone prefer a Gel battery over an AGM?
Gel batteries are more suitable for situations where deep discharges are frequent. They can handle deeper discharge levels without immediate damage and are more resistant to cold temperatures. If someone plans to discharge their battery over 50% of its capacity regularly, a Gel battery might be a better choice.
How does temperature affect these batteries?
Gel batteries are more resistant to cold temperatures compared to AGM batteries. If you’re storing or using batteries in colder environments, Gel batteries might be more appropriate.
Are AGM batteries more affordable than Gel batteries?
Yes, AGM batteries are generally less expensive than Gel batteries. However, it’s essential to consider the specific needs and long-term benefits before making a purchase based solely on price.
How does the design age of Gel batteries impact their performance?
Gel batteries were developed in the 1970s, making their design a bit outdated. There are limited engineering options left to improve them, which might make them less adaptable to newer technologies or applications compared to AGM batteries.
Can both AGM and Gel batteries be installed in any orientation?
Yes, both AGM and Gel batteries can be installed in any angle or orientation, making them versatile for various applications and installations.
Final Thoughts
AGM and gel batteries are both great options for storing energy, but they have different benefits and drawbacks. Ultimately, the best battery for you will depend on your specific needs. Be sure to consider all of the factors involved before making a decision. Doing so will save you time and money.
AGM batteries are an excellent option for a reliable and durable battery. They are perfect for vehicles that frequently experience start-ups and heavy use, such as boats, RVs, or cars. They also perform well when feeding power-hungry appliances.
Gel batteries offer some advantages over AGMs — they can withstand deeper discharge levels and have a longer life span. However, they don’t perform as well for high current draws and are more expensive than AGM batteries.
Now that you know the advantages and disadvantages of AGM vs. Gel batteries, which one is right for you?

